From Correction to Prevention: How CAPA Software Dashboards Drive Continuous Improvement
November 11, 2025
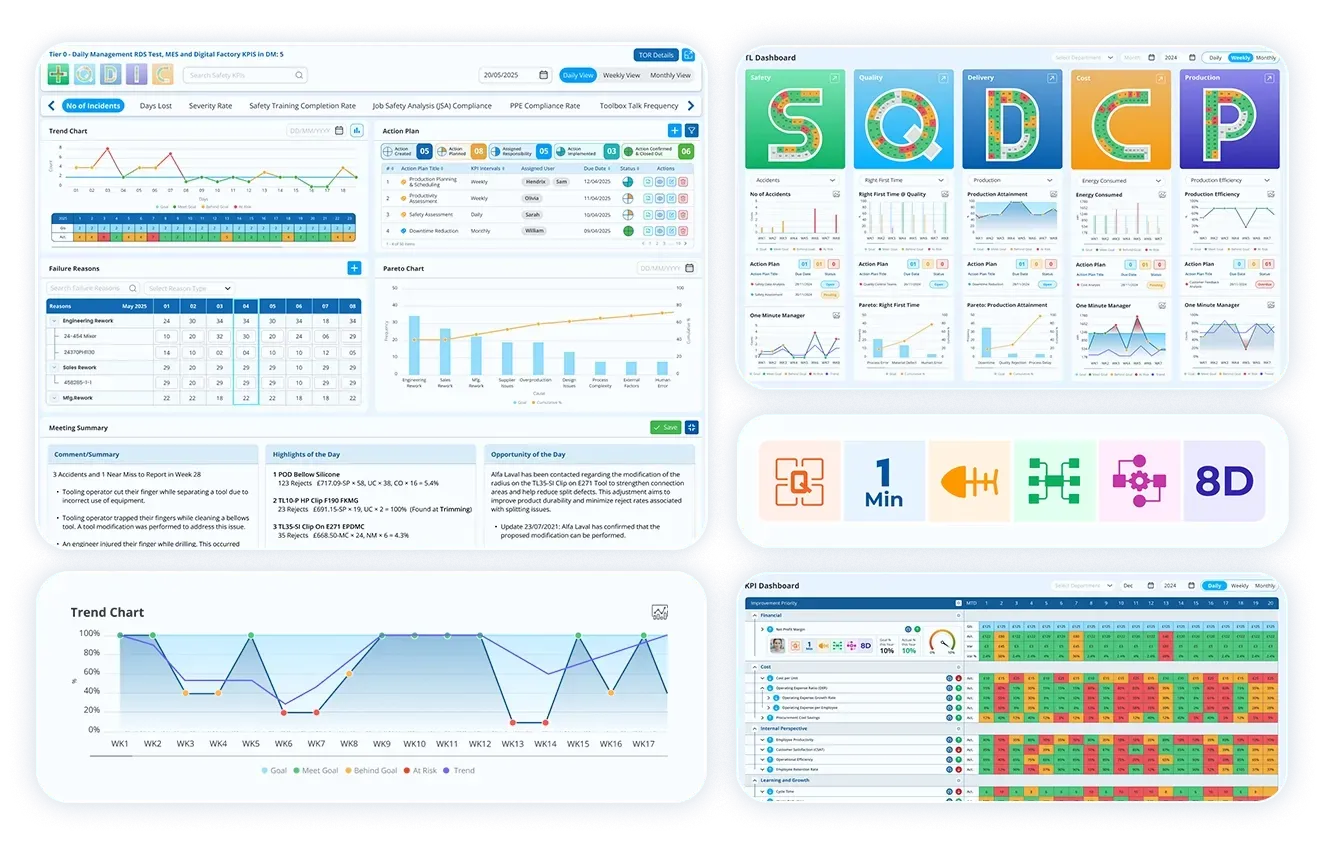
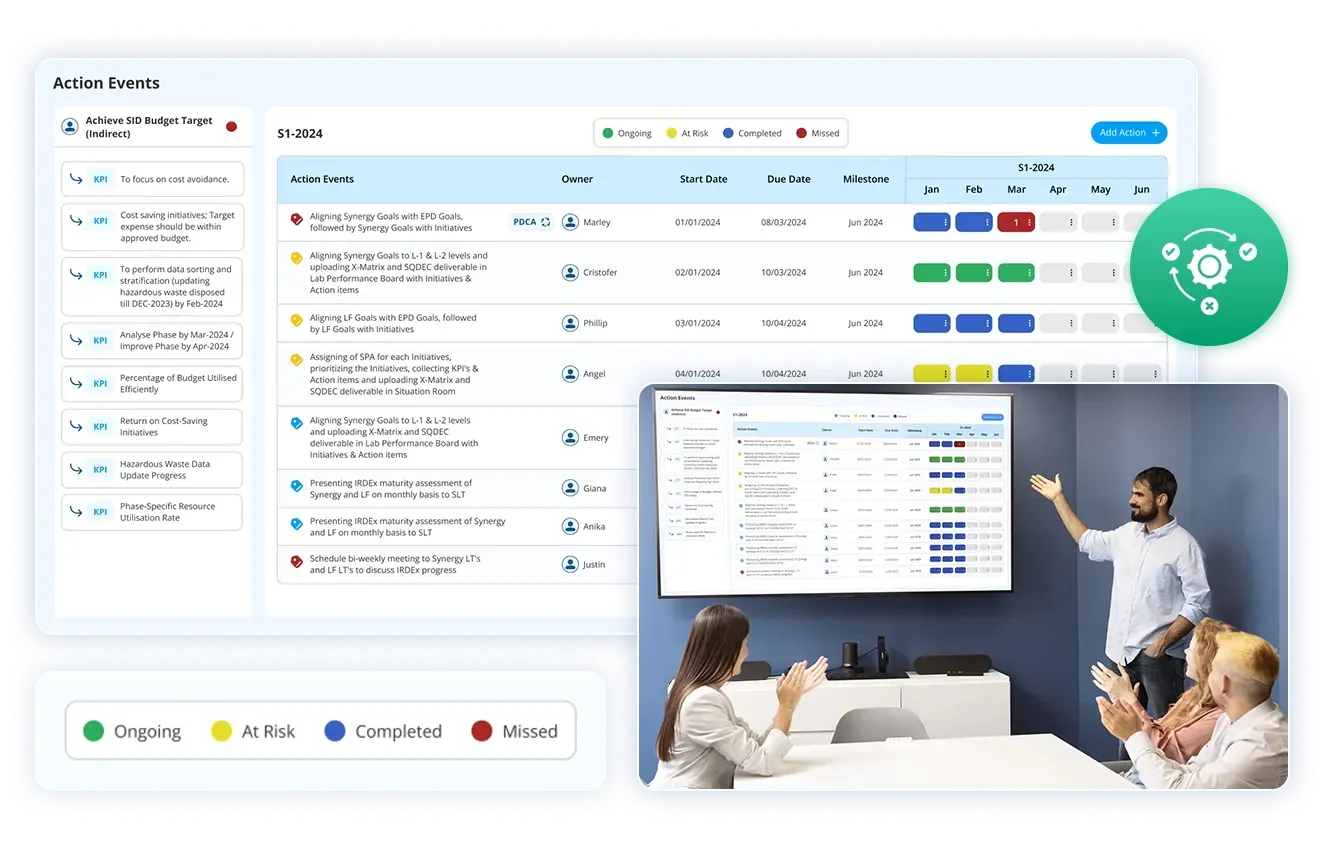
In today’s fast-moving and quality-driven industries, it is important to guarantee consistent compliance, and continuous improvement is more serious than ever. Corrective and Preventive Action (CAPA) management software plays a vital role in helping organisations identify, address, and prevent issues that could impact product quality, safety, or regulatory compliance. By automating and streamlining the CAPA process, this software equips teams to respond swiftly to discrepancies, analyse root causes effectively, and execute long-term solutions. This blog further explores what CAPA management software is, how it functions, and why it has become a crucial tool for businesses committed to operational excellence.
What is CAPA?
The acronym CAPA stands for Corrective and Preventive Action. CAPA is a structured approach used in Quality Management Systems (QMS) to identify, inspect, and put an end to causes of issues (discrepancies) and prevent their recurrence or occurrence.
It is one of the most vital components of continuous improvement