The pulse of perfection: 7 Quality metrics Pharma plants must monitor in real-time
Last updated on : July 30, 2025
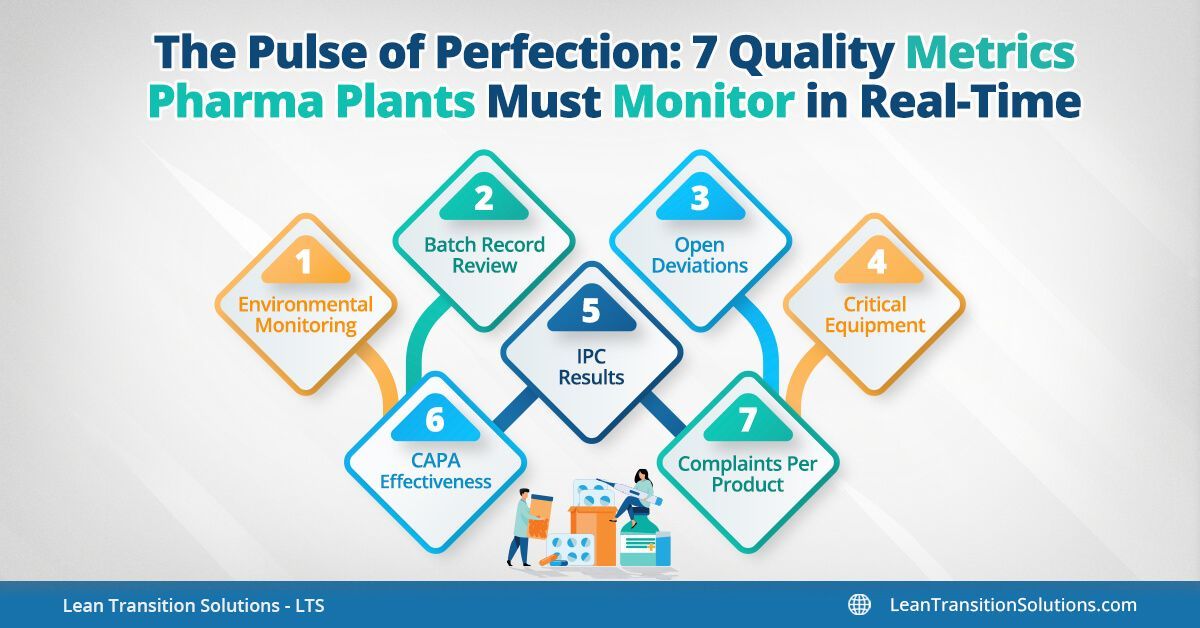
Pharma plants monitor a wide array of quality indicators daily including - batch failure rates, CAPA timelines, deviation closures, and yield percentages to Right-First-Time (RFT), complaint trends, equipment downtime, environmental conditions, OOS/OOT events, and audit readiness scores.
But only a handful demand real-time visibility to catch issues early, stay compliant, and drive continuous improvement. In this blog, we spotlight 7 critical pharma quality metrics that must move beyond static tracking — and be embedded into live dashboards, alerts, and automated workflows for true operational control.
Why are tracking quality metrics significant in Pharmaceutical industry?
Quality is the backbone of every pharmaceutical plant. From raw material checks to final product release, every stage depends on monitoring the right metrics at the right time. Yet too often, data on critical factors like batch yield, contamination risks, or deviations is reviewed only after the fact — when problems have already turned into losses or compliance gaps.
To maintain product integrity and meet strict GMP requirements, pharma teams need to track key quality indicators continuously, not just during audits or routine checks. Real-time visibility into these metrics helps identify risks early, drive corrective actions faster, and build a culture of continuous improvement, all supported by the right digital tools that bring quality data together in one place.